
Debunking Misconceptions About Cantron
Misinformation spreads quickly online—and unfortunately, Cantron is no exception. As part of the family of formulas originating from the late chemist James Vincent Sheridan’s inspired discovery, Cantron has been unfairly grouped with products making unapproved disease claims, misrepresented by critics, and dismissed without a true understanding of its purpose or effectiveness.
This article sets the record straight and offers clarity for those seeking the truth about Cantron’s origins, safety, and role in a modern wellness protocol.
-
Cantron Is a Dietary Supplement—Not a Drug
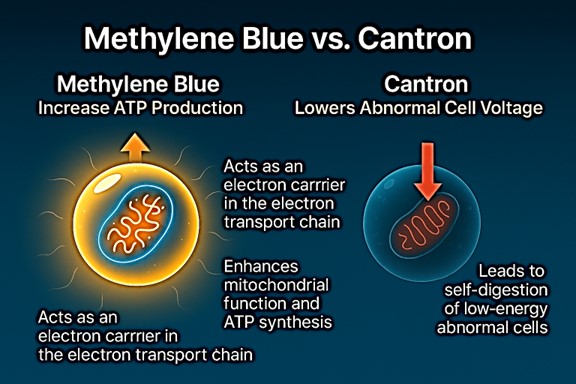
Cantron is a nutraceutical dietary supplement designed to support bioelectrical cellular health and overall wellness. Medical Research Products, its manufacturer, has never claimed Cantron treats or cures any specific disease. The formula is offered only as a dietary supplement and should be used to support the body’s own natural healing processes.
While other versions of the Sheridan formula may have crossed regulatory lines with health claims, Cantron has always tried to remain within legal and ethical boundaries, promoting the product as part of a broader wellness program.
-
Cantron’s Origins Are Truly Unique
James Vincent Sheridan, a brilliant chemist, credited divine inspiration for the development of this formula. He refused payment or royalties, calling the formula a gift for humanity. Over the years, several versions of his formula have been produced, including Entelev, Cancell, Protocel, and Cantron.
Among these, Cantron stands out as the most concentrated, researched, and potent version, thanks to advanced manufacturing techniques and in-depth in-vitro studies by the Josephine Ford Cancer Research Center in Detroit Michigan.
-
Negative Online Reports Often Refer to Other Versions
Many online criticisms stem from confusion with other products like Cancell, which were tested by the National Cancer Institute (NCI) under protocols that were inappropriate for evaluating non-toxic nutraceuticals. The NCI used a 48-hour test model—best suited for chemotherapy drugs—when Sheridan had recommended a 7-day protocol for the formula to exhibit full activity.
In his October 15, 1990 letter to the NCI, Jim Sheridan refers to sending the material to be tested and reminds them that the formula has no toxicity to normal cells, indicating that this formula should not be tested in a model for toxic drugs. In his letter he corrects a mistatement from NCI’s October 2, 1990 letter. He writes, “For the record, may I correct a mistatement in your letter? You say your tests of over 10 years ago determined the compound to be inactive. This statement is incorrect. In your tests you did something wrong. I called it to your attention. You repeated the test (5) additional times. Despite my repeated letters and phone calls, you repeated the error all five (5) times. Running the test incorrectly six (6) times does not justify your conclusion. ” Despite Sheridan’s letter, the NCI did just what Sheridan warned against and the conclusion was just as Sheridan predicted.
In the NCI’s abbreviated trial, 58 cancer cell lines were tested. Even in the flawed 48-hour study, three of those cancer cell lines showed 100% tumor regression and every other cell line had some degree of tumor regression. According to the Journal of The Bio-Electro-Magnetics Institute, Volume 3, Number 4, March 1993, 50 of the 58 cell lines had a 50% or better result, 31 cell lines decreased by 80 % or better, and 15 cell lines had a 95% or better result, yet the NCI declared the material inactive once again. Author Dr. John Zimmerman points out that Cancell had many more “hits” (over 80% tumor regression) than Taxol, the chemotherapy drug of choice at that time While that data was ignored in final summaries, the facts tell a different story.
According to Louise Trull’s book, “The Cancell Cointroversy,”Sheridan believed that if the correct protocol for non-toxic substaces would have been followed, all the cell lines would be completely be eliminated and all lines would have -100% growth. Cantron, when tested at the Josephine Ford Cancer Research Center under the proper protocols, complete tumor regressions were demonstrated in all 13 cell lines tested within 3–5 days, offering scientific validation that other versions never received. This study was published in a peer reviewed journal.
CURRENT TOPICS IN NUTRACEUTICAL RESEARCH Vol. 11, No. 1/2, pp. 9-14, 2013
ISSN 1540-7535 print, Copyright © 2013 by New Century Health Publishers, LLC
www.newcenturyhealthpublishers.com
All rights of reproduction in any form reserved
Disclaimer: The FDA has not reviewed this article. This article is for information purposes only. Cantron is a dietary supplement with powerful antioxidant properties. No claims for the cure, prevention or mitigation of any disease are made or implied! According to the Dietary Supplement Act of 1994, dietary supplement manufacturers and distributors may disseminate articles from peer-reviewed journals and from complete chapters in books which have information about their products if those articles and chapters are not part of the label. This article in fact is from a peer-reviewed journal and not made part of our label. MRP reserves the right to publish this article.
Additional in-vitro tests were conducted by Southern Research another reputable laboratory located in Birmingham, alabama. Using a 3-day paradigm, it discovered 100% tumor regression on two other cancer cell lines that were tested. This test produced a surprising result. While Cancer cells developed a resistance to chemotherapy, remarkably there was no resistance to Cantron.
For those keeping score, 15 out of 15 cancer cell lines were tested and all 15 cell lines had 100% tumor regression with Cantron when tested under the paradigm for a non-toxic substance, and cancer cells did not develop a resistance to Cantron as it did with cytotoxic chemotherapeutic drugs. And as previously mentioned Cancell was more active then the chmotherapy drug of choice. This controversy needs to be settled once and for all. I will be attempting to contact the new administration, and in the spririt of the M.A.H.A. movement request that they test all 58 cell lines at NCI using the proper protocol.
Since Cantron is not intended to diagnose, cure, mitigate, or prevent any disease, and since positive in-vitro findings only indicate potential (animal studies and clinical trials are needed before FDA approval and before any disease claims can be made) why is it important to correct the record? Because truth matters and our reputation matters. It is a matter of wrongfully sullying Cantron’s reputation, and wrongly categorizing it as a Quack remedy. This has caused our company immense harm, and we have the right to defend the truth. NCI has a powerful voice that resonates throughout the entire medical establishment, who republish this faulty information all over the Internet. Legal action against all of these players has not been ruled out.
-
Misunderstandings Are Often Amplified by Skeptics and Misinformation Websites
Sites that list “quack” cures often fail to differentiate between products, instead lumping all versions together with sweeping accusations. These critics seldom perform original research or speak to those who have used Cantron successfully.
Additionally, some forums and blog posts republish the same outdated or misinformed content without checking facts—leading to a cycle of misinformation that’s hard to break.
-
We Support an Informed Public, Not a Misled One
Cantron users deserve accurate information. That’s why we are publishing this blog. We are committed to transparency and integrity—and to countering misinformation with facts, and the real science.
In Conclusion: Don’t Let Misinformation Stand in the Way of Your Wellness
Whether you’re a newcomer to Cantron or a longtime advocate, understanding the facts is essential. Cantron is not a drug. It is not a cure. But it is a safe, powerful, and well-tolerated antioxidant-based supplement that has supported thousands on their wellness journey for over 42 years.
Explore our site to learn more, or reach out to us directly if you have questions.
➡ Learn about our wellness philosophy
➡ Explore Cantron’s Total Wellness Program
➡ Understand the Origins of the Sheridan Formulas
➡ View published in-vitro studies
References:C
- CURRENT TOPICS IN NUTRACEUTICAL RESEARCH Vol. 11, No. 1/2, pp. 9-14, 2013
ISSN 1540-7535 print, Copyright © 2013 by New Century Health Publishers, LLC
www.newcenturyhealthpublishers.com - Letter from Departmernt of Health and Human Services, Ven L Newman, PhD, National Cancer Institute to James V Sheridan, October 2, 1990
- Letter from James V Sheridan to Departmernt of Health and Human Services, Ven L Newman, PhD, National Cancer Institute, October 15, 1990.
- Journal of The Bio-Electro-Magnetics Institute, Volume 3, Number 4, March 1993
- The Cancell Controversy, Luise B Trull, Hampton Roads Publising Company, 1993
- Southern Research, Bimingham, Alabama
![]()




Recent Comments